TIM-3 therapy for Alzheimer’s is emerging as a promising avenue in the battle against this debilitating disease. Recent studies have shed light on the role of this immune checkpoint molecule, suggesting that its inhibition could liberate microglia— the brain’s immune cells—allowing them to effectively remove amyloid plaques that are notorious for disrupting cognitive function. The innovative approach has not only shown potential in diminishing plaque accumulation but also indicates cognitive improvement in experimental models. As Alzheimer’s treatment strategies evolve, the emphasis on the TIM-3 gene could mark a pivotal shift toward harnessing the body’s immune system to combat neurodegeneration. With increasing evidence supporting this method, TIM-3 therapy could redefine our understanding of treating Alzheimer’s disease and providing hope for millions affected.
The exploration of TIM-3 therapy as a solution for Alzheimer’s disease introduces an intriguing intersection of immunology and neurodegenerative disorders. This innovative treatment focuses on manipulating immune checkpoint molecules to enhance the brain’s ability to clear harmful plaques, a hallmark of Alzheimer’s. By targeting the TIM-3 gene, researchers are investigating ways to boost the function of microglia, potentially leading to significant cognitive improvements. As traditional Alzheimer’s treatments often fall short, leveraging the body’s own immune mechanisms may pave the way for more effective therapeutic strategies. With its potential to transform Alzheimer’s care, TIM-3 therapy represents a cutting-edge approach to addressing one of healthcare’s greatest challenges.
Understanding TIM-3 and Its Role in Alzheimer’s Disease
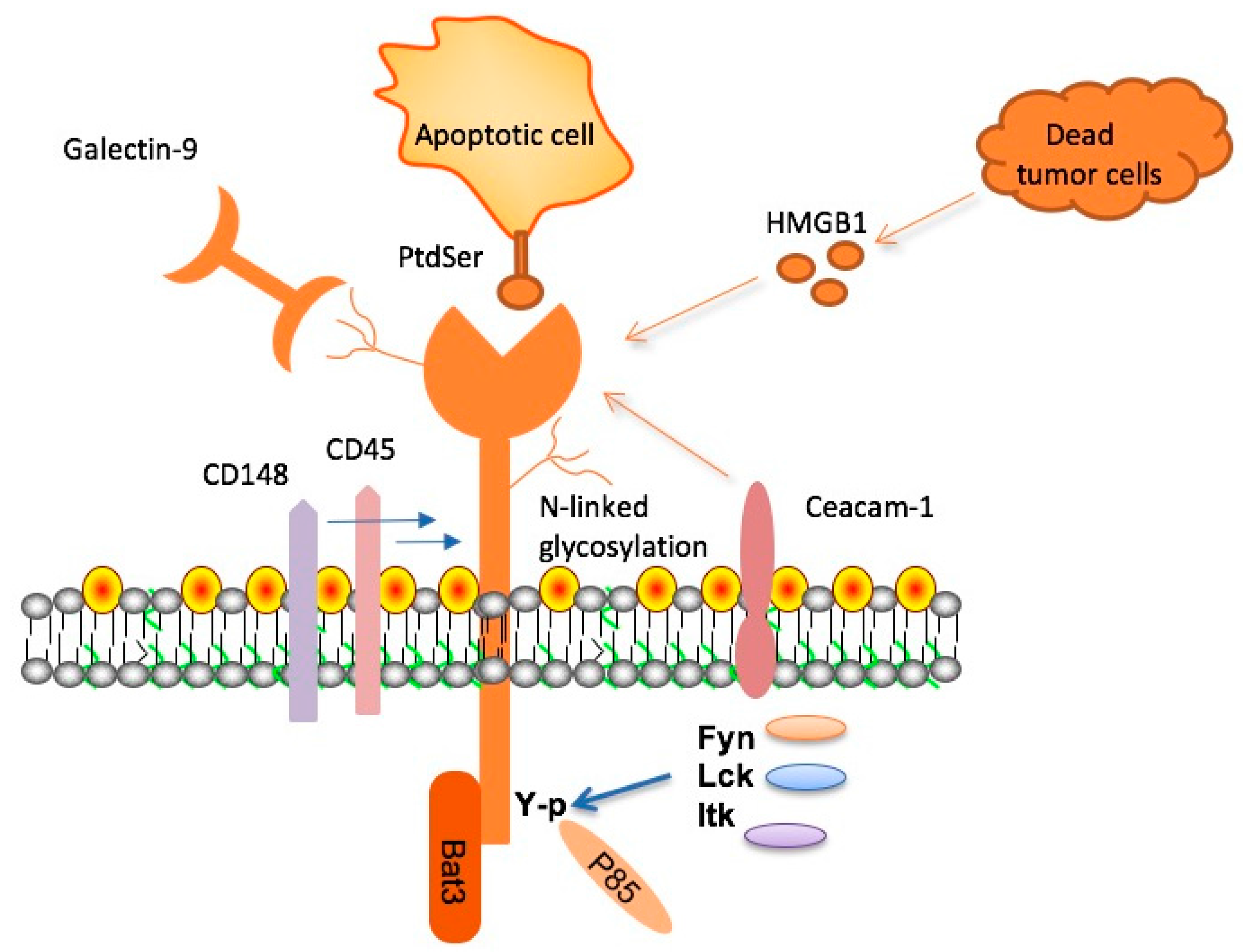
TIM-3, a notable immune checkpoint molecule, has recently gained attention in Alzheimer’s treatment research due to its crucial role in regulating microglial activity. Microglia are the brain’s resident immune cells, tasked with cleaning up amyloid plaques associated with Alzheimer’s disease. Under normal circumstances, these cells respond to the buildup of plaques, but with the high expression of TIM-3, their ability to clear these harmful deposits is severely inhibited. This inhibition not only leads to increased plaque accumulation but also contributes to cognitive decline, making TIM-3 an important target for therapeutic intervention.
Research indicates that TIM-3’s role as a checkpoint molecule is similar to its function in cancer, where it prevents T cells from damaging healthy tissue. In the context of Alzheimer’s, the high levels of TIM-3 expression in microglia result in what can be considered a dysfunctional state where the immune response is dampened. By deleting the expression of the TIM-3 gene in experimental models, researchers observed a notable increase in microglial activity against plaques, suggesting that TIM-3 therapy for Alzheimer’s could potentially enhance cognitive function by restoring microglial function.
The Mechanism of TIM-3 Therapy for Alzheimer’s
TIM-3 therapy for Alzheimer’s aims to reverse the inhibitory effects of TIM-3 on microglia, thereby restoring their ability to clear amyloid plaques. Researchers propose using anti-TIM-3 antibodies or small molecules as a form of treatment. These agents would block the inhibitory signals sent by TIM-3, empowering microglia to resume their plaque-clearing functions. This strategy is particularly promising given the genetic links between TIM-3 and late-onset Alzheimer’s disease, where a polymorphism in the TIM-3 gene has been associated with increased risk.
In experiments involving genetically modified mice lacking the TIM-3 gene, researchers observed significant cognitive improvements as microglia effectively engulfed amyloid plaques. This underscores the potential of TIM-3 therapy to not only mitigate cognitive impairments caused by plaque accumulation but also to alter the nature of the plaques themselves. Compacted plaques are less harmful and lead to improved cognitive function, which illustrates how targeting TIM-3 could revolutionize Alzheimer’s treatment landscapes.
Evaluating Cognitive Improvement in Mouse Models
The use of animal models, particularly mice, is foundational in evaluating the potential effectiveness of TIM-3 therapy for cognitive improvement in Alzheimer’s. In studies where the TIM-3 gene was deleted, researchers reported enhanced memory and navigation skills in the modified mice compared to their non-modified counterparts. These findings suggest that when microglia are freed from the constraints imposed by TIM-3, they are capable of restoring cognitive functionality, which is often severely impaired in Alzheimer’s due to plaque accumulation.
Cognitive testing in mice is typically carried out through tasks that measure their memory and ability to navigate complex environments, such as mazes. In one notable study, mice burdened with amyloid plaques exhibited impaired spatial navigation and diminished memory capacity. After TIM-3 was deleted, these mice displayed behaviors linked to normal cognitive function, such as increased exploratory behavior and reduced anxiety in open spaces. This evidence supports the hypothesis that TIM-3 therapy not only aids in plaque clearance but also reinstates vital cognitive processes critical for the survival and well-being of the organism.
Implications of TIM-3 Research for Alzheimer’s Treatment
The implications of TIM-3 research for Alzheimer’s treatment are profound, particularly as traditional therapies have faced limitations and setbacks. With an estimated 90% of Alzheimer’s cases being late-onset, discovering therapeutic avenues that address the underlying pathology of plaque formation is crucial. The recent studies exploring TIM-3 reflect a shift towards targeting the immune system components involved in Alzheimer’s pathogenesis. By understanding and manipulating molecules like TIM-3, researchers are paving the way for innovative treatments that leverage the body’s immune response to combat cognitive decline.
Excitingly, repurposing existing anti-TIM-3 antibodies that have been previously studied in cancer therapy may provide a shortcut to testing these therapies in humans. Recent advancements in therapeutic strategies denote a potential boom in Alzheimer’s treatment options, where TIM-3 therapy could theoretically lead to significant improvements in both plaque reduction and cognitive function restoration in patients. Exploiting the immune system’s natural capabilities presents a hopeful frontier in the fight against Alzheimer’s, emphasizing the importance of continued research in this field.
Comparative Studies of TIM-3 and Other Checkpoint Molecules
In the landscape of Alzheimer’s treatment, understanding the distinctions and similarities between TIM-3 and other immune checkpoint molecules is key to developing effective therapies. While TIM-3 is a focus in current research, other checkpoint molecules like PD-1 and CTLA-4 are also being studied for their roles in modulating immune responses in various diseases. Each molecule offers unique pathways for therapeutic intervention, highlighting the importance of tailored approaches in Alzheimer’s treatment.
Additionally, the comparative study of TIM-3 with other immune checkpoint molecules provides valuable insights into how these inhibitors function differently in neurological contexts versus cancer. For example, while PD-1 has shown promise in reversing T-cell exhaustion in tumors, TIM-3’s role in Alzheimer’s may revolve more around microglial behavior and plaque clearance. This nuanced understanding could lead to combination therapies that target multiple checkpoints, potentially leading to synergistic effects in cognitive improvement.
Future Directions in TIM-3 and Alzheimer’s Research
As researchers continue to explore TIM-3’s role in Alzheimer’s pathology, future directions may include testing various anti-TIM-3 compounds in humanized mouse models that better mimic human disease conditions. Efforts are underway to determine how the human TIM-3 variant behaves in relation to amyloid beta deposition and cognitive impairment. These studies are critical for translating findings from animal models to human applications, where efficacy and safety must be thoroughly evaluated.
Moreover, ongoing collaboration between laboratories specializing in neuroimmunology and therapeutic development will be essential for optimizing TIM-3 therapies. The pathway to clinical trials will involve rigorous testing to understand the ideal dosing, administration routes, and potential side effects of targeting TIM-3 in Alzheimer’s patients. The ultimate goal is to bring forth an innovative treatment that could significantly alter the course of Alzheimer’s disease, offering hope to millions affected by this devastating condition.
The Genetic Basis of TIM-3 in Alzheimer’s Disease
The genetic contributions of TIM-3 to Alzheimer’s disease through polymorphisms in the HAVCR2 gene present a fascinating area of research. Studies suggest that variations in this gene can significantly impact how TIM-3 functions in microglial cells. Those carrying specific polymorphisms may exhibit heightened risk for Alzheimer’s due to altered immune responses and reduced capability to clear amyloid plaques, making genetic profiling an essential aspect of understanding individual susceptibility to the disease.
Furthermore, investigating the genetic landscape surrounding TIM-3 can provide insights into potential biomarkers for Alzheimer’s. By identifying genetic risk factors that influence microglial activation and plaque formation, researchers might uncover new therapeutic targets or strategies to personalize Alzheimer’s treatment. This genetic approach, when integrated with TIM-3 therapy, could enhance predictive capabilities in disease progression and response to treatment, ultimately fostering a more tailored approach to Alzheimer’s management.
Challenges and Considerations in TIM-3 Therapy Development
While TIM-3 shows great promise for Alzheimer’s treatment, several challenges and considerations must be addressed before clinical application. Understanding the long-term effects of inhibiting TIM-3 is crucial, as prolonged immune activation can lead to unintended consequences, including neuroinflammation. The balance between stimulating microglial activity and avoiding excessive inflammation will be a critical factor in designing TIM-3 therapies.
Additionally, ensuring that the therapeutic approach targets the brain effectively is vital. Since the blood-brain barrier presents significant obstacles for many therapeutic agents, optimizing the delivery mechanisms for anti-TIM-3 antibodies to reach the central nervous system is paramount. These considerations underscore the complexity of developing TIM-3 therapies and highlight the need for interdisciplinary collaboration in neuroscience, immunology, and pharmacology to overcome potential barriers and ensure successful treatment outcomes.
Patients’ Perspectives on Novel TIM-3 Therapies
Patients’ perspectives play a crucial role in the development and acceptance of novel treatments like TIM-3 therapies for Alzheimer’s. Understanding patients’ experiences with traditional therapies, their preferences for treatment options, and their willingness to participate in new clinical trials can guide researchers in crafting more effective communication and treatment strategies. Patient-centric approaches ensure that the development of preventive and therapeutic measures is aligned with what patients find beneficial.
Engaging with the community through educational initiatives about TIM-3 and its role in Alzheimer’s treatment could also foster greater awareness and involvement in clinical trials. Feedback from patients not only aids researchers in refining their approach but also empowers patients by giving them a voice in their treatment journeys. As the landscape of Alzheimer’s therapy evolves with emerging research like TIM-3, understanding the human experience behind the science becomes ever more essential.
Frequently Asked Questions
What is TIM-3 therapy for Alzheimer’s disease and how does it work?
TIM-3 therapy for Alzheimer’s disease involves targeting the TIM-3 checkpoint molecule, which inhibits the brain’s immune cells (microglia) from clearing amyloid plaques. By blocking the inhibitory action of TIM-3, the microglia are reactivated to attack and remove these plaques, potentially leading to cognitive improvement in individuals with Alzheimer’s.
How effective is TIM-3 therapy for improving cognitive functions in Alzheimer’s patients?
While studies on TIM-3 therapy for Alzheimer’s are still in early stages, research has shown that deleting TIM-3 in mouse models leads to improved memory and cognition. This suggests that TIM-3 therapy may have the potential to enhance cognitive functions in Alzheimer’s patients by enabling microglia to clear harmful plaques.
What role do immune checkpoint molecules like TIM-3 play in Alzheimer’s disease?
In Alzheimer’s disease, immune checkpoint molecules like TIM-3 prevent microglia from effectively removing toxic amyloid plaques. This accumulation of plaques is linked to cognitive decline, as TIM-3 inhibits the microglia’s phagocytic ability, which is essential for maintaining brain health and function.
Can TIM-3 therapy be used in conjunction with other Alzheimer’s treatments?
Yes, TIM-3 therapy could potentially be used alongside other Alzheimer’s treatments. As research progresses, combining TIM-3 blockade with existing therapies may provide a comprehensive approach to managing Alzheimer’s, particularly in improving the immune response against plaque accumulation.
What are the implications of TIM-3 gene polymorphisms in Alzheimer’s disease?
Polymorphisms in the TIM-3 gene (HAVCR2) have been associated with an increased risk of late-onset Alzheimer’s disease. These genetic variations may influence the expression of TIM-3 on microglia, affecting their ability to clear amyloid plaques, and thereby contributing to the progression of Alzheimer’s.
What are the next steps for TIM-3 therapy research in Alzheimer’s treatments?
Future research on TIM-3 therapy for Alzheimer’s includes testing the efficacy of human anti-TIM-3 antibodies in mouse models of the disease. Successful trials may pave the way for clinical applications, helping to halt the development of plaques and restore cognitive functions in Alzheimer’s patients.
Is TIM-3 therapy exclusively for Alzheimer’s disease or can it be used for other conditions?
While TIM-3 therapy is currently being explored for Alzheimer’s disease, checkpoint inhibitors like TIM-3 have broader applications in other diseases, particularly cancers. The research may reveal that similar immune modulation strategies could benefit various neurodegenerative and autoimmune conditions.
What distinguishes TIM-3 therapy from traditional Alzheimer’s treatments?
Unlike traditional Alzheimer’s treatments that primarily focus on symptomatic relief, TIM-3 therapy aims to address the underlying disease mechanisms by enhancing microglial activity to clear amyloid plaques, potentially slowing disease progression and leading to tangible cognitive improvement.
| Key Points | Details |
|---|---|
| Introduction of TIM-3 Therapy for Alzheimer’s | A new study suggests that TIM-3 therapy, an immune-system strategy used in cancer treatment, may improve cognitive function in Alzheimer’s patients. |
| Role of TIM-3 | TIM-3 is a checkpoint molecule that inhibits microglia, the brain’s immune cells, from clearing amyloid plaques that accumulate and harm brain function. |
| Prevalence of Late-Onset Alzheimer’s | 90% to 95% of Alzheimer’s cases are late-onset, linked to high expression of TIM-3. |
| Impact on Microglia and Plaques | By deleting TIM-3, mice models showed enhanced microglial clearance of plaques and improved cognitive behavior. |
| Future Applications of TIM-3 Therapy | Development of anti-TIM-3 antibodies could potentially halt plaque development in Alzheimer’s patients based on mouse model studies. |
Summary
TIM-3 therapy for Alzheimer’s shows promise as a novel treatment strategy that utilizes immune checkpoint inhibition to enhance the brain’s ability to clear harmful amyloid plaques. By deleting TIM-3, researchers have observed significant cognitive improvements in mouse models, paving the way for potential human therapies. If further studies confirm these findings, anti-TIM-3 treatments could revolutionize Alzheimer’s care, providing hope to millions affected by this debilitating condition.