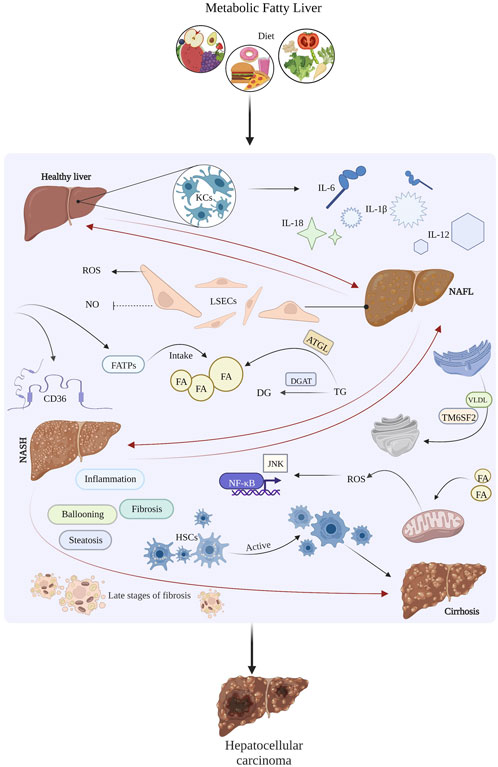
Liver cancer bile imbalance has emerged as a critical area of focus in understanding hepatocellular carcinoma, the most prevalent form of liver cancer. Recent studies reveal that disruptions in bile acid balance can significantly contribute to liver disease, leading to severe consequences such as inflammation and fibrosis. The role of bile acids extends beyond digestion; they act as hormonal messengers that influence metabolic processes in the liver. Researchers have identified a key molecular switch that affects bile acid regulation, which could unveil new avenues for treatment. Understanding how the FXR and YAP pathways interact in this imbalance offers promising insights into combating liver cancer.
The imbalance of bile in the context of liver health is intricately linked to the development of liver malignancies, particularly hepatocellular carcinoma. Bile, crucial for fat digestion, also plays a significant role in metabolic regulation, affecting how the liver responds to various stimuli. Disruptions in bile acid homeostasis can lead to heightened inflammation and liver injury, paving the way for more severe liver conditions. This research highlights the complex interactions between bile acids, liver function, and cancer pathways, specifically focusing on the roles of FXR and the YAP pathway. By exploring these relationships, we can better understand and potentially mitigate the impacts of liver disease.
Understanding the Link Between Bile Acid Imbalance and Liver Cancer
Bile acid imbalance can significantly impact liver health, particularly contributing to the development of liver diseases such as hepatocellular carcinoma (HCC). This imbalance disrupts the delicate homeostasis of bile acids, which are crucial for effective digestion and metabolism. The liver’s production of bile acids serves various functions, including aiding in the emulsification of fats and serving as signaling molecules that influence metabolic pathways. When the careful regulation of these bile acids is disturbed, it can lead to toxic accumulation and result in liver inflammation, fibrosis, and ultimately cancer.
Recent research has indicated that a critical factor in this process is the molecular switch involving the farnesoid X receptor (FXR). FXR regulates bile acid synthesis and transport. When the balance is tipped—often due to the overactivity of the YAP pathway—the function of FXR is compromised, leading to excess bile production. This creates a scenario where liver cells are damaged and can begin to transform into cancerous cells. Understanding these pathways opens the door to potential treatment strategies focused on restoring bile acid balance.
Role of the YAP Pathway in Liver Disease Progression
The Hippo/YAP pathway plays a pivotal role in regulating various cellular functions, including growth and metabolic control. In liver disease, the YAP pathway has been shown to act as a double-edged sword; while it is essential for normal cell regeneration, its overactivation can provoke liver pathologies, including hepatocellular carcinoma (HCC). Particularly, YAP’s interaction with FXR is crucial, as its activation can inhibit FXR’s regulatory function, leading to an accumulation of bile acids. This disruption not only contributes to liver inflammation but also propels the progression toward malignancy.
Researchers have found that inhibiting YAP’s repressor function could be a promising therapeutic approach to prevent liver cancer. By enhancing FXR activity or promoting the excretion of bile acids, it is possible to mitigate the impacts of this imbalance. These findings shine a light on the potential for pharmacological interventions that target the YAP pathway, offering a therapeutic avenue for patients at risk for liver diseases associated with bile acid dysregulation.
Therapeutic Implications of FXR Activation
Activating FXR represents a new frontier in the fight against liver diseases, particularly in situations where bile acid imbalance leads to conditions such as hepatocellular carcinoma. Researchers have demonstrated that compounds which stimulate FXR can significantly improve liver health by restoring bile acid homeostasis. When FXR is activated, it not only enhances the normal processing and secretion of bile acids but also reduces inflammation and fibrosis in the liver, thereby lowering the risk of cancer progression.
Additionally, experimental models have shown that pharmacological agents targeting FXR can counteract the deleterious effects of YAP overactivity. By focusing on these molecular mechanisms, future therapies could effectively address both the underlying causes of bile acid imbalance and its catastrophic outcomes—leading to safer, more effective management of liver cancer and other related diseases.
Impacts of Bile Acid Accumulation on Metabolic Health
The accumulation of bile acids due to imbalances in their production or excretion has profound implications not only for liver health but for overall metabolic regulation. Excess bile acids can cause liver injury and inflammation, and their presence in elevated quantities may interfere with metabolic signaling processes, potentially leading to conditions like insulin resistance and metabolic syndrome. This metabolic disharmony underscores the importance of understanding bile acid dynamics in the context of liver and systemic health.
Moreover, the metabolic disturbances caused by bile acid accumulation can trigger a vicious cycle, exacerbating liver disease progression and increasing the risk of developing hepatocellular carcinoma. Therefore, research focusing on how to restore the normal circulation and metabolism of bile acids may lead to innovative strategies for combating not just liver cancer but a broader array of metabolic diseases.
Innovative Approaches in Cancer Research
The ongoing study of the relationship between bile acids, liver health, and cancer has opened avenues for innovative therapeutic approaches. Researchers are harnessing knowledge about the molecular mechanisms that govern bile acid metabolism to develop targeted treatments that might prevent liver diseases from advancing to cancer. This involves scrutinizing the roles of critical components like FXR and the YAP pathway and understanding how they regulate bile production and metabolism.
New drugs that can selectively activate FXR or inhibit YAP’s cancer-promoting functions could revolutionize treatments for patients with liver disease. Highlighting these avenues not only enhances our understanding of liver cancer biology but fosters the development of targeted, individualized treatment strategies that could significantly improve patient outcomes.
Future Directions in Liver Cancer Research
As researchers continue to unravel the complexities of liver cancer, the relationship between bile acids and cancer progression remains a critical area of focus. The identification of the molecular switch involving YAP and FXR presents exciting opportunities for new directions in liver cancer research. Future studies will likely focus on how to manipulate these pathways therapeutically to restore bile acid balance and diminish the risk of cancer.
In addition, understanding the broader implications of bile acid metabolism on overall health will be crucial. This research could pave the way for new screening methods or preventive strategies that identify individuals at higher risk for developing liver diseases due to bile acid imbalances, subsequently informing proactive management plans to mitigate liver cancer’s incidence.
The Role of Nutrient Sensing in Liver Health
Nutrient sensing is fundamental to maintaining liver health and metabolic balance. The liver plays a key role in regulating nutrient levels and responding to dietary inputs, and bile acids are significant players in this intricate system. By modulating nutrient sensing pathways, bile acids can impact various metabolic processes, from glucose metabolism to lipid regulation.
Recent findings suggest that disruptions in nutrient sensing, influenced by bile acid imbalance, can contribute to liver disease’s progression, including hepatocellular carcinoma. By studying how bile acids interact with nutrient sensing mechanisms, researchers aim to develop interventions that could enhance liver function and potentially protect against liver-related cancers, highlighting the importance of this area in future health research.
The Interconnectivity of Liver Health and Systemic Disorders
The health of the liver is intricately linked to broader systemic disorders. A compromised liver due to bile acid imbalance can lead to multiorgan dysfunction, affecting cardiovascular health, diabetes, and even neurological functions. This emphasizes the importance of understanding liver diseases not only in isolation but as part of an interconnected web of bodily health where bile acids play crucial roles.
Furthermore, addressing liver health can have far-reaching benefits for preventing systemic diseases. For instance, managing bile acid levels effectively could alleviate burdens in systemic disorders, showcasing the need for an integrated approach to treatment. This perspective can guide future research and clinical practices aimed at enhancing patient outcomes through comprehensive health strategies.
Exploring Non-Invasive Diagnostic Techniques
The development of non-invasive diagnostic techniques for assessing liver health and bile acid balance is a growing field of interest. Early detection of liver dysfunction linked to bile acid imbalances can significantly improve patient outcomes and provide critical insights into the risk of progressing to hepatocellular carcinoma. Innovative imaging techniques, biomarker analyses, and liquid biopsies are paving the way for more effective monitoring of liver health without the need for invasive procedures.
Implementing these non-invasive methods in clinical practice could revolutionize how liver diseases are diagnosed and managed. By providing real-time insights into bile acid dynamics and liver function, healthcare providers may be able to intervene earlier in disease progression, leading to more personalized and effective treatment strategies for at-risk populations.
Frequently Asked Questions
What role do bile acids play in liver cancer and bile imbalance?
Bile acids are crucial for digestion and metabolism. An imbalance in bile acids can trigger liver diseases, including hepatocellular carcinoma (HCC). When regulation of bile acid production is disrupted, it leads to liver injury and inflammation, ultimately increasing the risk of liver cancer.
How does the FXR receptor impact bile acid levels in liver cancer?
The Farnesoid X receptor (FXR) is essential for maintaining bile acid homeostasis. In liver cancer, the activation of the YAP pathway can inhibit FXR function, leading to an overproduction of bile acids, which contributes to liver disease and promotes the progression to hepatocellular carcinoma.
What is the significance of the YAP pathway in liver disease and bile acid metabolism?
The YAP pathway plays a critical role in regulating cell growth and bile acid metabolism. In liver cancer, activated YAP can repress FXR, resulting in bile acid imbalance that damages liver tissue and fosters inflammation, paving the way for hepatocellular carcinoma.
Can targeting bile acids improve liver cancer treatments?
Yes, targeting bile acid pathways by enhancing FXR function or promoting bile acid excretion could lead to new liver cancer treatments. By restoring the balance in bile acids, it may be possible to reduce liver damage and hinder the progression of liver cancer.
What are the potential therapeutic interventions involving FXR in liver cancer?
Potential therapeutic interventions include pharmacological stimulation of FXR, which is promising for halting bile acid overproduction. Other strategies might involve inhibiting the repressive functions of YAP or increasing bile acid export protein (BSEP) levels to restore bile acid homeostasis in liver cancer patients.
How does liver disease relate to bile acid homeostasis?
Liver disease often stems from bile acid imbalance, which can disrupt metabolic processes and lead to inflammation and fibrosis. This dysregulation is significantly associated with the development of hepatocellular carcinoma, highlighting the importance of maintaining proper bile acid levels for liver health.
What findings did recent research reveal about bile imbalance and liver cancer?
Recent research identified a key molecular switch that regulates bile acid metabolism, showing that its disruption can lead to liver injury and increase the risk of hepatocellular carcinoma. The study’s insights into YAP and FXR pathways offer potential new avenues for treating liver cancer.
What lifestyle changes can help manage bile acid imbalance linked to liver diseases?
Managing bile acid imbalance associated with liver diseases may involve dietary adjustments, such as reducing fatty foods, increasing fiber intake, and maintaining a healthy weight. Regular monitoring and medical advice are also key to managing liver health effectively.
| Key Point | Description |
|---|---|
| Bile Imbalance | Imbalance in bile acids can trigger liver diseases, including liver cancer. |
| Hepatocellular Carcinoma (HCC) | The most common form of liver cancer linked to bile acid regulation issues. |
| YAP Protein | A key molecular switch that represses bile acid regulatory functions. |
| FXR (Farnesoid X receptor) | A nuclear receptor crucial for bile acid homeostasis, inhibited by YAP. |
| Research Implications | Enhancing FXR function or reducing YAP activity could lead to new treatments for liver cancer. |
| Future Directions | Potential for pharmacological solutions that stimulate FXR to combat liver cancer. |
Summary
Liver cancer bile imbalance is increasingly recognized as a critical factor in the progression of liver diseases, particularly hepatocellular carcinoma (HCC). Recent research highlights how disruptions in bile acid levels, governed by the YAP protein and the FXR receptor, can lead to liver injury and cancer. Understanding the mechanisms of bile acid regulation opens avenues for developing targeted therapies that could significantly improve treatment outcomes for patients suffering from liver cancer.